Prospective, non-randomized Clinical Trial
A Dutch National Study on behalf of the Center for Personalized Cancer Treatment (CPCT) to Facilitate Patient Access to Commercially Available, Targeted Anti-cancer Drugs to determine the Potential Efficacy in Treatment of Advanced Cancers with a Known Molecular Profile

This is a prospective, non-randomized clinical trial that aims to describe the efficacy and toxicity of commercially available, targeted anticancer drugs prescribed for treatment of patients with advanced cancer with a potentially actionable variant as revealed by a genomic or protein expression test. The study also aims to simplify patient access to approved targeted therapies that are contributed to the program by collaborating pharmaceutical companies and to perform next generation sequencing on tumor biopsies for biomarker analyses. Eligible patients have an advanced solid tumor, multiple myeloma or non-Hodgkin lymphoma for which standard treatment options are no longer available and acceptable performance status and organ function. A genomic or protein expression test must have been performed on the tumor and the results must identify at least one potentially actionable molecular variant as defined in the protocol. Results from the molecular profiling test will be used to determine an appropriate drug(s) from among those available in the protocol. The choice of drug will be supported by a list of potential profiles, a molecular tumor board, a knowledge library and by study coordinators for review and approval of the match. The protocol-specified treatment will be administered to the patient once any drug-specific eligibility criteria are confirmed and a fresh pre-treatment biopsy is performed for future biomarker studies. All patients who receive treatment with a drug available in the protocol will be followed for standard efficacy outcomes including tumor response, progression-free and overall survival as well as duration of treatment. In addition, treatment related toxicity will be evaluated.
OBJECTIVES
Primary objectives
To describe the anti-tumor activity and toxicity of commercially available, targeted anti-cancer drugs used for treatment of patients with an advanced solid tumor, multiple myeloma or non-Hodgkin lymphoma that harbours a genomic- or protein expression variant known to be a drug target or to predict sensitivity to a drug.
To facilitate patient access to commercially available, targeted anti-cancer drugs of potential efficacy prescribed for treatment of patients with an advanced solid tumor, multiple myeloma or non-Hodgkin lymphoma that harbours a genomic- or protein expression variant known to be a drug target or to predict sensitivity to a drug.
Secondary objective
To perform refined biomarker analyses, including (but not limited to) next generation sequencing, on a fresh tumor biopsy specimen.
DRUP European Collaborations
DRUP European Collaborations
In 2023, two European projects were launched: PCM4EU (Personalized Cancer Medicine for all EU citizens), funded by the EU4Health program as part of Europe’s Beating Cancer Plan (grant: 101079984) and PRIME-ROSE (Precision Cancer Medicine Repurposing System Using Pragmatic Clinical Trials), funded under the Horizon Europe program (grant: 101104269).
These projects aim to broaden access for patients to precision cancer medicine, by building a network of trials that based their protocol on the original Drug Rediscovery Protocol (DRUP) from the Netherlands, so-called DRUP-like clinical trials. This European network of investigator-initiated clinical trials that have an aligned trial design and primary endpoints, facilitates accelerated accrual and data sharing, thereby improving patients access to off-label treatments. There are currently five ongoing European DRUP-like clinical trials, besides DRUP, and five are planning to start soon.
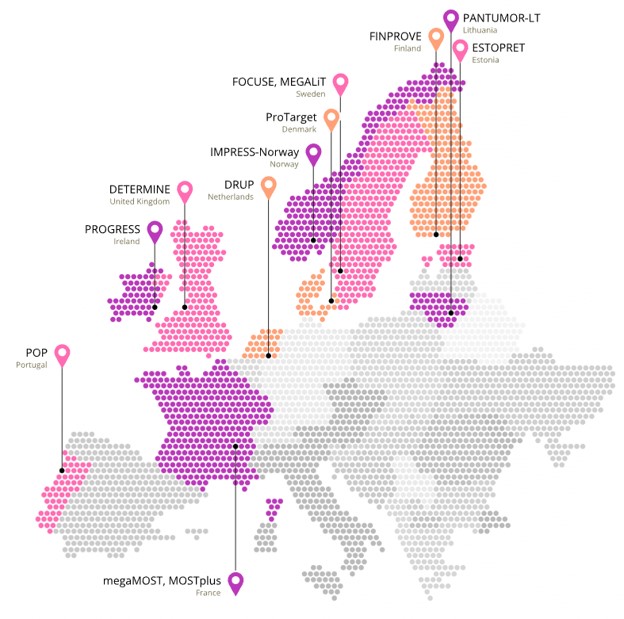
PCM4EU, that started in January 2023 and will continue until June 2025, focuses on the existing standards in molecular testing, from the availability of biomarker tests to implementation of clinical decision support systems and interpretation of results. With the formation of a data sharing platform, PCM4EU provides the basis for pooled cohort analyses between the different DRUP-like trials within PRIME-ROSE, that started in July 2023 and will run for 5 years. The PRIME-ROSE project will also strengthen methodologies in precision cancer clinical trials by e.g., developing synthetic control arms, developing models for the standardization of clinical data, and analyzing frameworks for the evaluation and reimbursement of on- and off-label oncology drugs in European countries. A detailed article that highlights the different activities in both projects can be found here.
For more information on the ongoing European projects, please follow the links below:
http://www.pcm4eu.eu/
http://www.prime-rose.eu/


