DRUP -The Drug Rediscovery Protocol (DRUP trial)
A Dutch National Study on behalf of the Center for Personalized Cancer Treatment (CPCT) to Facilitate Patient Access to Commercially Available, Targeted Anti-cancer Drugs to determine the Potential Efficacy in Treatment of Advanced Cancers with a Known Molecular Profile.
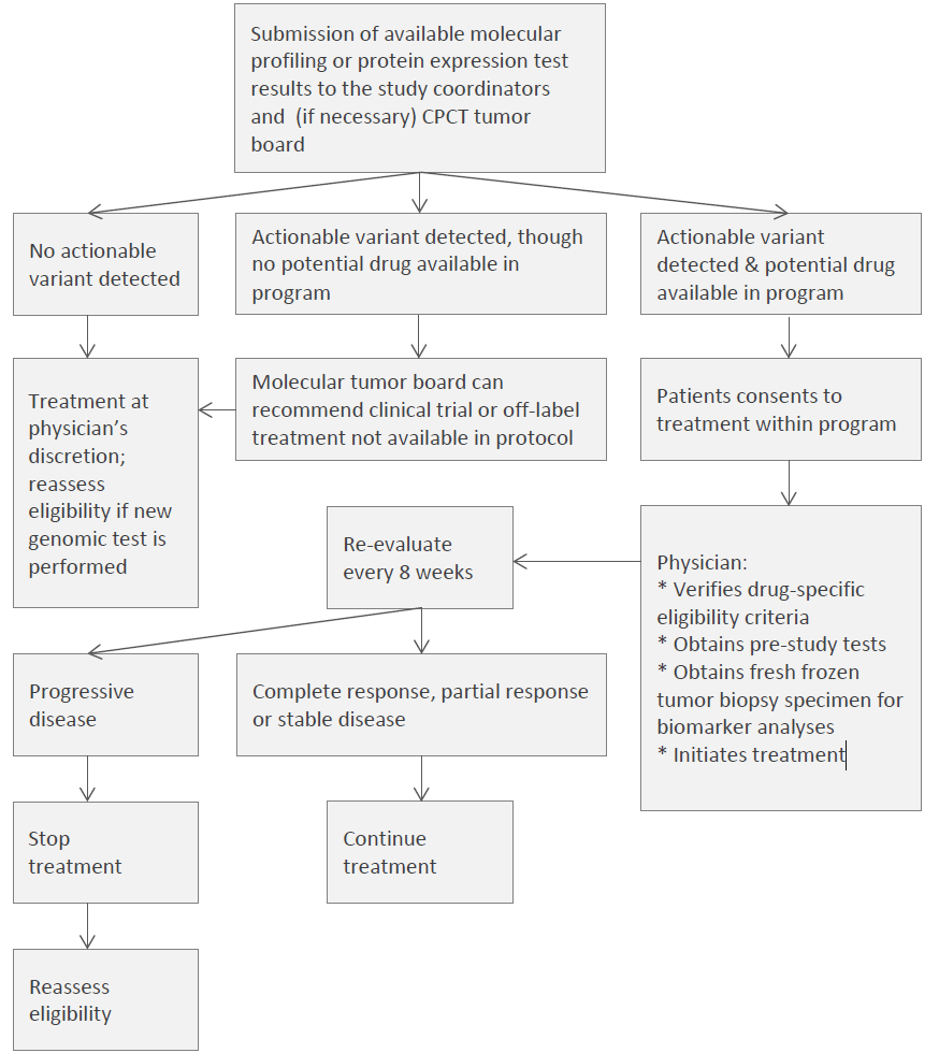
This is a prospective, non-randomized clinical trial that aims to describe the efficacy and toxicity of commercially available, targeted anticancer drugs prescribed for treatment of patients with advanced cancer with a potentially actionable variant as revealed by a genomic or protein expression test. The study also aims to simplify patient access to approved targeted therapies that are contributed to the program by collaborating pharmaceutical companies and to perform next generation sequencing on tumor biopsies for biomarker analyses. Eligible patients have an advanced solid tumor, multiple myeloma or non-Hodgkin lymphoma for which standard treatment options are no longer available and acceptable performance status and organ function. A genomic or protein expression test must have been performed on the tumor and the results must identify at least one potentially actionable molecular variant as defined in the protocol. Results from the molecular profiling test will be used to determine an appropriate drug(s) from among those available in the protocol. The choice of drug will be supported by a list of potential profiles, a molecular tumor board, a knowledge library and by study coordinators for review and approval of the match. The protocol-specified treatment will be administered to the patient once any drug-specific eligibility criteria are confirmed and a fresh pre-treatment biopsy is performed for future biomarker studies. All patients who receive treatment with a drug available in the protocol will be followed for standard efficacy outcomes including tumor response, progression-free and overall survival as well as duration of treatment. In addition, treatment related toxicity will be evaluated.
Objectives:
- Primary objectives
- To describe the anti-tumor activity and toxicity of commercially available, targeted anti-cancer drugs used for treatment of patients with an advanced solid tumor, multiple myeloma or non-Hodgkin lymphoma that harbours a genomic- or protein expression variant known to be a drug target or to predict sensitivity to a drug.
- To facilitate patient access to commercially available, targeted anti-cancer drugs of potential efficacy prescribed for treatment of patients with an advanced solid tumor, multiple myeloma or non-Hodgkin lymphoma that harbours a genomic- or protein expression variant known to be a drug target or to predict sensitivity to a drug.
- Secondary objective
- To perform refined biomarker analyses, including (but not limited to) next generation sequencing, on a fresh tumor biopsy specimen.
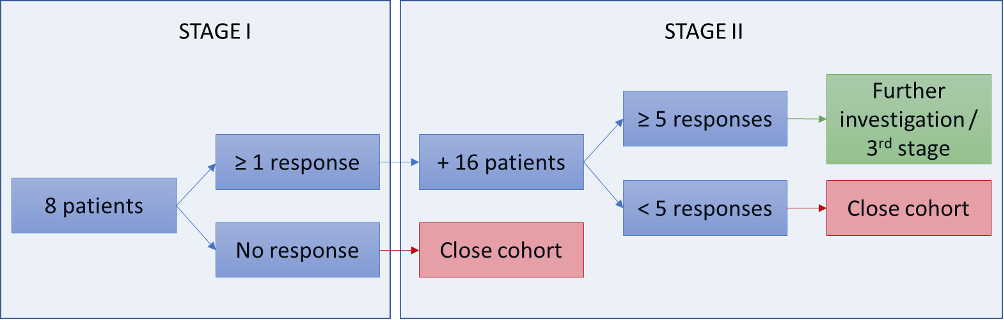
Cohort design stage I and II
Per cohort, 8 or 24 patients can be enrolled depending on response (defined as ‘response’ individually per criteria, or as disease control (absence of disease progression) for at least 16 weeks measured ≥2x and ≥28 days apart), as depicted below (figure 2). Each tumor type/variant/drug treatment will define a separate cohort, and each cohort will be monitored using a Simon-like two-stage ‘admissible’ monitoring plan to identify tumor/variant/drug cohorts with evidence of activity. For purposes of cohort definition, the ‘variant’ category will be defined at the level of the gene that harbors the mutation or overexpression.